
Patient portrayal.
Individual results may vary.
Side effects
TRINTELLIX® (vortioxetine) is a prescription medicine used in adults to treat a certain type of depression called Major Depressive Disorder (MDD). TRINTELLIX has not been shown to be safe and effective for use in children.

Common side effects of TRINTELLIX
You may experience side effects with TRINTELLIX. The most common side effects in short-term clinical studies were nausea, constipation, and vomiting.
Constipation
Vomiting
Nausea was usually considered to be mild or moderate, and its frequency was dose related. Nausea generally occurred in the first week and became less frequent over time, usually lasting for about 2 weeks. Nausea may continue in some people.
Suicidal thoughts and actions and antidepressant drugs
TRINTELLIX and other antidepressant medications carry a Boxed Warning due to risk for suicidal thoughts and actions.
Specifically, you should know:
- TRINTELLIX and other antidepressants increase the risk of suicidal thoughts and actions in people 24 years of age and younger, especially within the first few months of treatment or when the dose is changed.
- TRINTELLIX is not for use in children under 18.
- Patients taking TRINTELLIX should call their doctor or get emergency help right away if they have new or sudden changes in mood, behavior, thoughts or feelings, or symptoms that are new, worse, or worry them; or if they develop suicidal thoughts or actions.
For more information on side effects, visit our full FAQ.

Sexual side effects caused by antidepressants are a common problem.
You're not alone. Many commonly used antidepressants have sexual side effects. In fact, people who have experienced sexual side effects say they are some of the most troublesome side effects of their depression treatment.
Antidepressants may cause sexual side effects, including:
- Lack of sexual drive
- Difficulty becoming aroused
- Difficulty achieving orgasm

Can TRINTELLIX cause antidepressant sexual side effects?
As with other antidepressants, TRINTELLIX can cause sexual side effects during treatment. In short-term clinical studies, up to 5% of people with MDD taking TRINTELLIX voluntarily reported having sexual side effects.
During seven short-term (6- to 8-week) studies evaluating the efficacy of TRINTELLIX on overall MDD symptom relief, the impact of TRINTELLIX on sexual side effects was also observed and recorded in those with normal sexual functioning before starting TRINTELLIX. The findings from these studies are shown below.
Sexual side effects reported by females and males on TRINTELLIX vs. sugar pill:
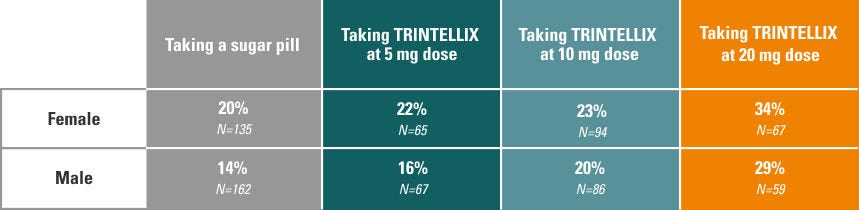
Sexual side effects with antidepressants are not always reported in clinical studies, partly because people may be uncomfortable discussing them. A patient questionnaire was used in TRINTELLIX clinical studies to better understand how many people without sexual problems at the beginning of the study experienced sexual side effects while taking TRINTELLIX.
Sexual problems:
Taking antidepressants like TRINTELLIX may cause sexual problems.
Symptoms in males may include:
- Delayed ejaculation or not being able to ejaculate
- Decreased sex drive
- Problems getting or keeping an erection
Symptoms in females may include:
- Decreased sex drive
- Delayed orgasm or not being able to have an orgasm
Talk to your healthcare provider if you develop any changes in your sexual function or if you have any questions or concerns about sexual problems during treatment with TRINTELLIX.
See below for Important Safety Information, including Boxed WARNING for Suicidal Thoughts and Actions.
Can changing to TRINTELLIX from certain antidepressants help improve sexual side effects?
In an 8-week clinical study, MDD patients whose depressive symptoms improved but who were experiencing sexual dysfunction on sertraline (ZOLOFT®), citalopram (CELEXA®), or paroxetine (PAXIL®) were switched to either TRINTELLIX or escitalopram (LEXAPRO®).
The clinical study showed that:

People who switched to TRINTELLIX showed greater improvement in antidepressant sexual side effects compared to people who switched to escitalopram (LEXAPRO®)*

People who switched to either TRINTELLIX or escitalopram (LEXAPRO®) maintained their improvement in depression symptoms from their previous antidepressant
*Based on an average change from the overall score on a standardized sexual functioning questionnaire.
Individual results may vary.
Concerned about weight gain?
TRINTELLIX did not have a significant impact on weight in short-term studies and during a 6-month phase of a long-term study of patients who responded to TRINTELLIX when compared to patients taking a sugar pill.
Some reports of weight gain have been received since product approval and also in a separate long-term study.
Important Safety Information
TRINTELLIX may cause serious side effects, including:
- Serotonin syndrome
- Increased risk of bleeding
- Hypomania (manic episodes)
- Discontinuation syndrome (side effects if you suddenly stop taking TRINTELLIX)
- Visual problems
- Low levels of salt in your blood
- Sexual problems
Please see additional Important Safety Information below.
TRINTELLIX is a prescription medication used in adults to treat Major Depressive Disorder (MDD). TRINTELLIX has not been shown to be safe and effective for use in children.
In multiple 6-8 week studies and one maintenance study vs. sugar pill, TRINTELLIX was shown to help reduce multiple symptoms of MDD based on an overall score on a standardized depression rating scale.
Individual results may vary.
Don’t be afraid to talk to your doctor.
Many people are reluctant to talk to their doctors about their antidepressant sexual side effects. While it can be an uncomfortable topic, you shouldn’t struggle in silence. Let your healthcare professional know about any side effects you’re experiencing.
Frequently Asked Questions
Before taking TRINTELLIX, tell your healthcare professional:
- about all your medical and other health conditions
- if you are pregnant or plan to become pregnant, since TRINTELLIX may harm your unborn baby. Taking TRINTELLIX during your third trimester may cause your baby to have withdrawal symptoms after birth or to be at increased risk for a serious lung problem at birth. Tell your doctor right away if you become or think you are pregnant while taking TRINTELLIX
- if you are breastfeeding or plan to breastfeed, since it is not known if TRINTELLIX passes into your breast milk
Stay connected
Sign up for tips, advice, information and patient stories to help you on your treatment journey.
